MARK D. MERUEÑAS, GMANews.TV
06/24/2009 07:30 PM
MANILA, Philippines –
Prices of flu drugs and vaccine have surged up to three times their normal prices, aggravating the Philippines’
Influenza A(H1N1) crisis as demand from health-conscious buyers is making the product so expensive.
The Philippines, which is lagging behind other countries in the race for securing ample supplies of anti-swine
flu vaccine, has listed 604 confirmed cases and one A(H1N1)-related fatality so far. It is now under pressure to take decisive steps in reducing mortality related to the novel virus.
Tamiflu – a popular brand of the
anti-viral drug oseltamivir used to treat symptoms of the A(H1N1) virus – now sells at P150.50 per capsule at a popular drugstore.
The
Department of Health (DOH) was able to purchase the anti-viral drug at much lower price before the A(H1N1) virus started spreading across the globe. Dr. Yolanda Oliveros, director of the DOH National Center for Disease Protection and Control, said the government bought its first batch of 750,000 capsules of Tamiflu at P40 each.
Another batch of 500,000 Tamiflu capsules was sold to the government through bidding at P48 a capsule after the Philippines started reporting A(H1N1) cases in the country, Oliveros said.
Since April, the DOH has distributed 600,000 Tamiflu capsules for free to patients who were infected by the new flu strain. All the medical expenses of A(H1N1) cases – from testing to hospitalization – have been shouldered by the DOH since the virus came out.
A source from the DOH told GMANews.TV that consumers have been calling the agency to inquire about the “high prices" of anti-flu
medicine and vaccines.
From P500 to P1,800
The exorbitant prices imposed by medical practitioners and drug firms may be considered “profiteering," and the government should find a way “to regulate" the prices of the flu products, said the source, who spoke on condition of anonymity.
A health advocacy group has called for the filing of charges against doctors and drug firms suspected of jacking up drug prices for Tamiflu and flu vaccines.
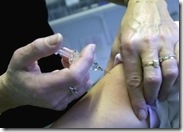
In an interview with GMANews.TV, Health Alliance for Democracy (HEAD) vice chairperson Gene Nisperos said the group has received reports about some doctors charging their patients as high as P1,800 for a flu shot, in addition to their professional fee (PF).
“Nakikinabang ang malalaking companies at sinasamantala nila ang takot ng mga tao [Big companies are benefiting from the scare and are taking advantage of the situation]," he said.
“Baka plus PF ay umabot na ng almost P3,000. Overpricing na iyan," (If the PF is included, the price would reach almost P3,000. That’s overpricing) he said.
A doctor who operates her own clinic in
Makati City admitted in an interview with GMANews.TV that she sells anti-flu vaccines – with brand names Agrippal, Fluarix, and
Vaxigrip – at P1,000 to P1,500 per dosage.
At a popular retail
drug store, GMANews.TV learned that a single dose of flu vaccine is sold at P695. Most doctors recommend one to two doses per person as protection from the seasonal flu virus.
According to Oliveros, the regular price of flu vaccine is only P500. The DOH is not undertaking free vaccination for the public, but has purchased vaccine for their employees, she said.
The DOH had earlier requested P93.5 million from the national government for “anti-A(H1N1) preparation," Oliveros said. The DOH has shelled out P60 million of the funds and intends to use the remaining amount to buy personal protective equipment such as masks.
Responsible pricing
Flu vaccine manufacturers and distributors are selling their products to drug retailers at “reasonable" prices, according to Dr. Sally Gatchalian, medical director for vaccines at the GlaxoSmithKline (
GSK). The company is a leading flu vaccine distributor in the Philippines, carrying the brand Fluarix.
Gatchalian told GMANews.TV that some patients probably get a steep bill for a flu shot because it includes expenses for medical examination, professional fee, and other miscellaneous fees.
GSK and other manufacturers of flu vaccine are members of the Pharmaceutical Health Care Association of the Philippines, which said it has no control and influence on the pricing policies of member firms.
“Pricing is an individual business decision of our member companies. We however encourage members to practice responsible pricing," the PHAP said in an official statement sent to GMANews.TV.
Vaccine supply running out
GSK and
Sanofi Pasteur have ran out of flu vaccine stocks amid the global health scare triggered by the spread of the A(H1N1) virus.
Sanofi Pasteur said its annual stock of flu vaccines usually lasts until September, but demand has doubled in recent months and the firm’s supply had dried up as early as June. GSK said its flu vaccine stocks had sold out much earlier in mid-May.
Dr. Remegio Olveda, director of the Regional Institute for Tropical Medicine, said the flu vaccines in the market only protect people from the regular seasonal flu viruses.
“Pero maganda na rin na protected ka sa seasonal flu para maiwasan na mag-mutate pa ang A(H1N1) virus," said Olveda. He stressed the possibility that the A(H1N1) virus, when introduced to another type of flu virus, could form a new strain.
A day after the
World Health Organization declared the onset of a global
flu pandemic last June 11, the Swiss drugs giant Novartis announced that it had developed an A(H1N1) vaccine. However, clinical
trials will only start in July, and the vaccine will not be available in the market until September, at the earliest. -
GMANews.TV
From GMANews.tv; see the source article here for the list of items and their recommended retail price.
 Image via Wikipedia
Image via Wikipedia